by Stephane Bancel*
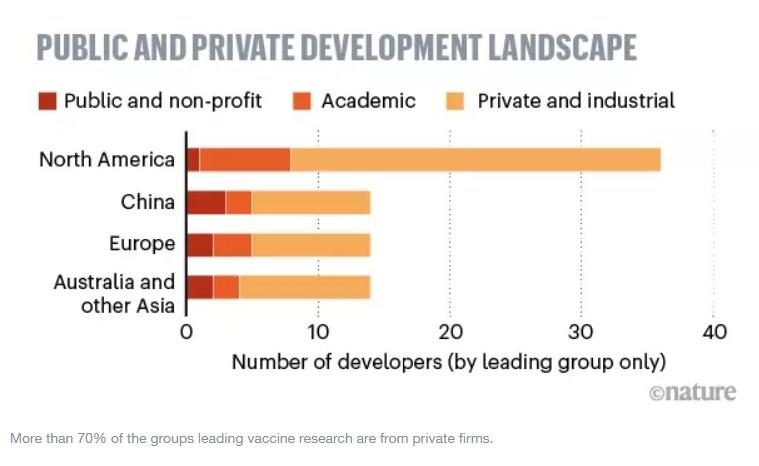
-There are 123 SARS-CoV-2 vaccines under development and seven that have entered human trials.
-Any potential vaccines need to be manufactured to scale in this pandemic setting.
-We must assess the potential speed with which a vaccine candidate can move towards approval.
In the face of an immense burden of disease and an unfathomable death toll from COVID-19, the world today has an intense need for hope. A degree of hope does come in some regions from diminishing numbers of cases, hospitalizations and deaths, caused by extreme restrictions on commerce, travel and social interaction.
Additional hope, sometimes ill-informed, comes from the development of potential therapeutics and vaccines. According to the Milken Institute, there are some 200 therapeutics being developed as possible treatments for COVID-19; and a remarkable 123 vaccines under development to prevent against infection by SARS-CoV-2, the virus responsible for causing COVID-19.
The company I lead, Moderna, Inc., is a clinical stage biotechnology company whose vaccine candidate against SARS-CoV-2, called mRNA-1273, was the first to enter human clinical trials in the United States, back on March 16. To date, this vaccine continues to progress positively through clinical trials. As such, a great deal of hope and public attention has been focused on our company and on this innovative potential medicine.
What I would like to do is to encourage you not to abandon hope in the context of the COVID-19 pandemic but ask that you tie your hope firmly to advancements in medical science. This will prevent you from reacting to well-meaning but potentially misguided enthusiasm or doubts. I will also suggest where specifically to look for validated elements of hope. My comments will focus on vaccines, since that is where my experience resides with respect to COVID-19.
My initial piece of guidance is this: be cautious of the sheer count of vaccine candidates. One hundred and twenty-three is a big number, but starting to develop a vaccine candidate is far different from bringing one to use in large populations. Our perspective at Moderna is that the only competitor we are racing against is the SARS-CoV-2 virus and the resulting devastation it brings. To us, more vaccines are welcome in this battle – and they are critical to addressing the level of human need around the world.
Don’t take false hope from the number of vaccine candidates; vaccines must move from development into clinical trials with humans, as an early step in progress towards eventual use. As of today, thankfully, there are at least seven vaccine candidates against the SARS-CoV-2 virus that have already entered human trials.
Beside ours, the six others come from the Beijing Institute of Biological Products, BioNTech (BNT162), CanSino Biologics (Ad5-nCoV), Inovio Pharmaceuticals (INO-4800), Sinovac Biotech, and University of Oxford (ChAdOx1 nCoV-19). This progress in a record timeframe is indeed encouraging – and a legitimate, medical reason for hope – but, as a number, seven is a long way from 123.
Now, within the set of potential vaccines that do make it into human trials, here are additional elements that I encourage you to watch for in order to engender true hope:
First, watch for specific early clinical indicators of safety and effectiveness. Vaccine candidates will typically be tested early on in animals, both to look for biological reactions that are consistent with appropriate immune system responses, as well as to see if the vaccine can prevent disease. Immune responses are assessed in the lab. Testing whether the vaccine can prevent disease will generally be done by challenging animals with the virus itself, and observing whether vaccinated animals actually avoid getting the disease.
Then, in humans, early trials will be conducted in relatively small numbers of healthy individuals. Those participants will be followed closely – first to assess safety and second to assess the production of neutralizing antibodies. Safety is relatively straightforward, since one is looking for the absence of serious adverse events. The presence of neutralizing antibodies indicates that the vaccine is creating substances that would be expected to defend a cell from the invading virus. In this case, it would be an indication that the vaccine may have rendered SARS-CoV-2 no longer infectious.
Next, to generate real hope, you must assess any potential vaccines in the context of whether they can be manufactured to scale in this pandemic setting. In the face of COVID-19, sponsors of vaccine candidates who cannot create product at the scale of tens or hundreds of millions of doses per month are probably not relevant in bolstering our hope at a global level. For our company, which has been previously in an R&D mode, recognizing this fact meant needing to execute a strategic production/supply agreement with a global partner that could rapidly amplify our volume to as much as a billion doses of vaccine per year.
Finally, the last element to secure hope is that you must assess the potential speed with which a vaccine candidate can move towards approval. In this case, I would be less concerned that a company has commercial rights to sell its vaccine, but more that relatively broad access to the vaccine may be granted to high-risk or other populations through widespread clinical trials, or through regulatory action by various governments. In the case with Moderna, we know that these regulatory paths are complex and, unless addressed early and with focus and scientific rigour, can create inadvertent delays in getting critical vaccines to vulnerable populations around the world.
My hope is that this message does not bring you undue concern about the potential for vaccines to prevent COVID-19, but instead gives you the tools to build legitimate confidence in where we and other companies are in combatting this pandemic. I can assure readers that I and my colleagues at Moderna share a humble confidence as we battle this enemy, and we approach every day with enthusiasm and unlimited energy in contributing in every way we can.
*Chief Executive Officer, Moderna
**first published in: www.weforum.org



 By: N. Peter Kramer
By: N. Peter Kramer
